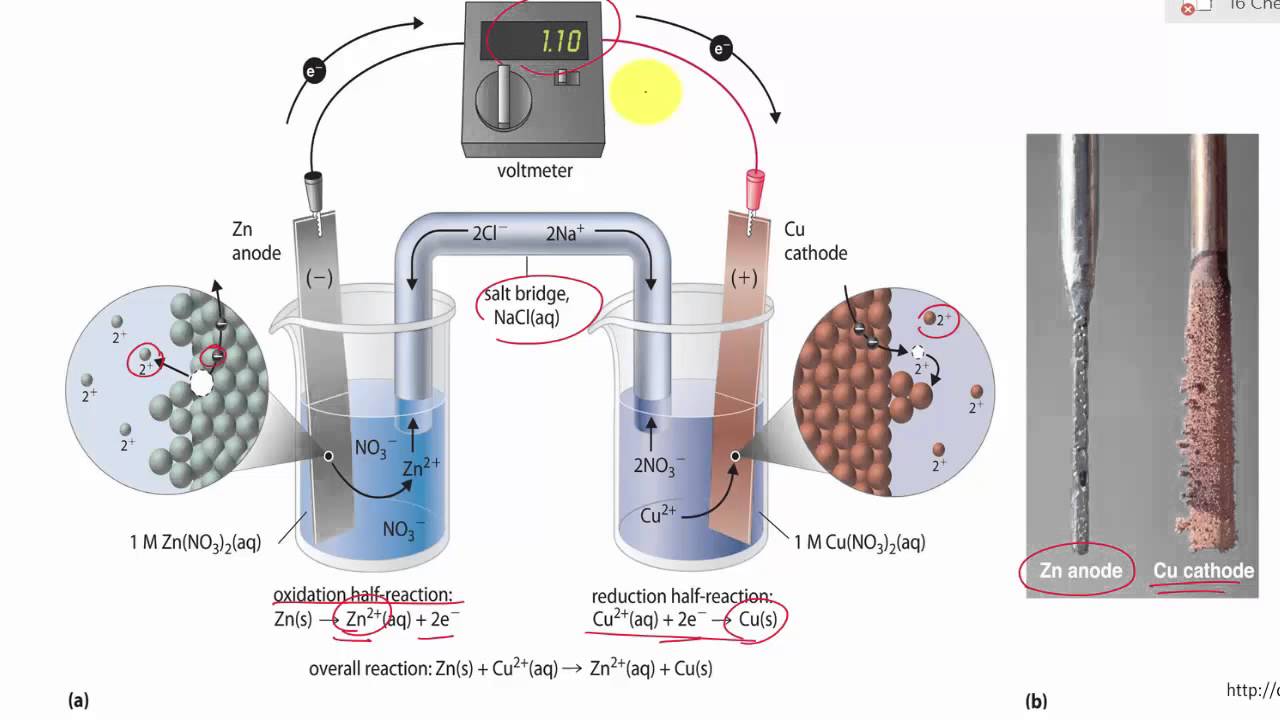
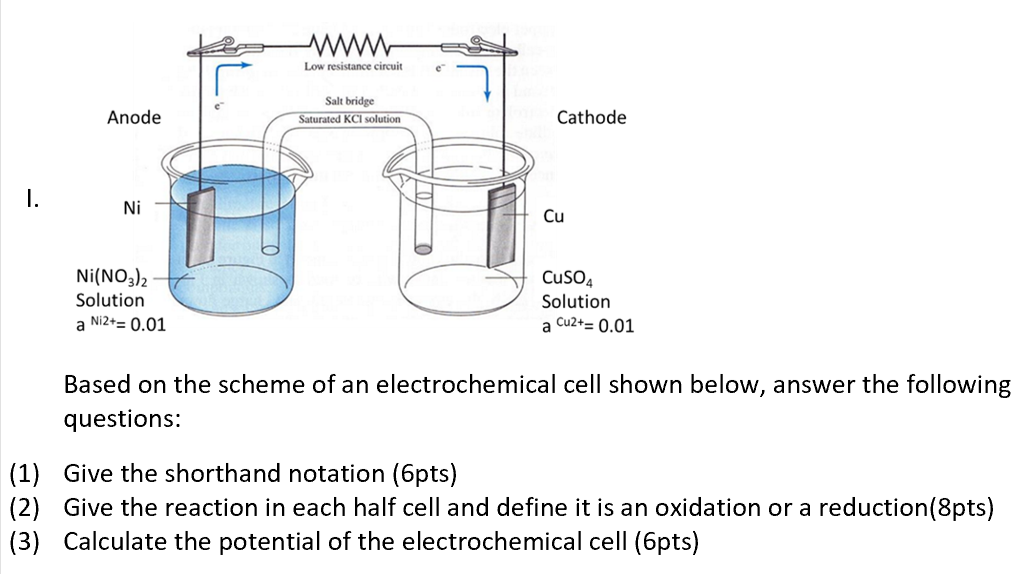
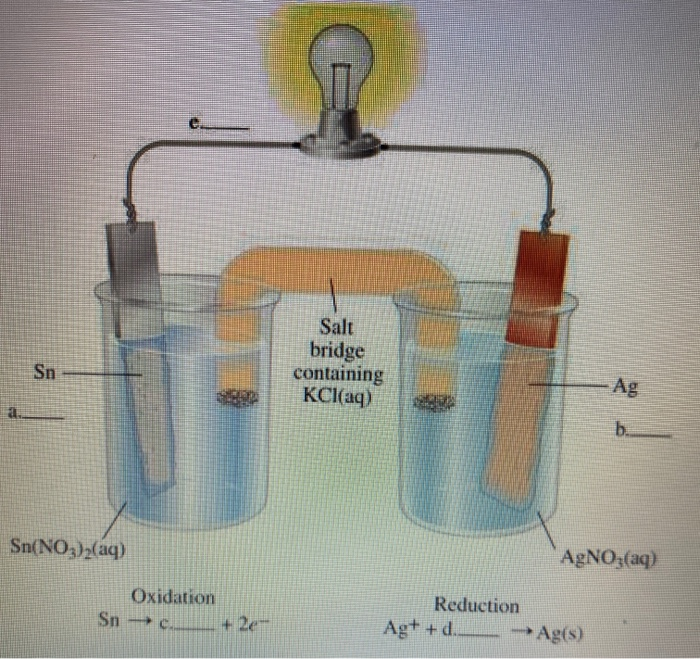
A reaction: 12H2(g) + AgCl(s) H^⊕(aq) + Cl^ (aq) + Ag(s) occurs in a galvanic cell. The structure of the cell will be:
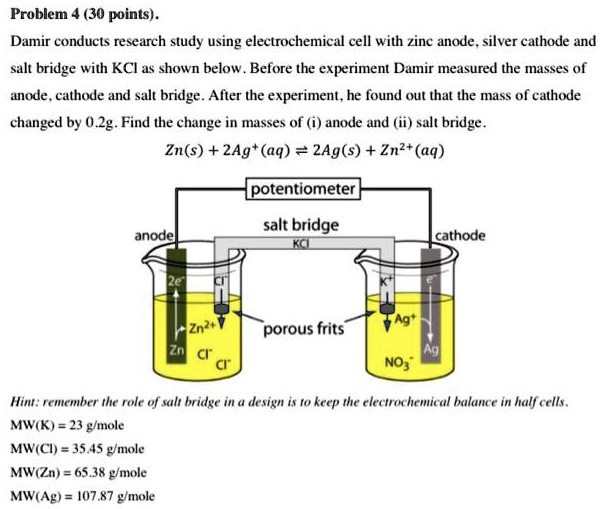
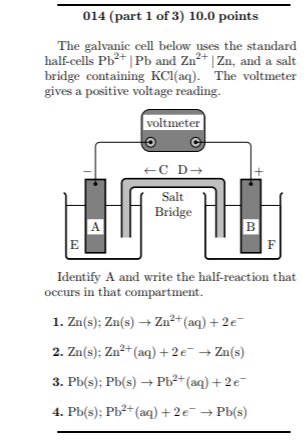
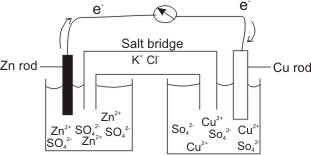
SOLVED: Problem 4 (30 points) . Damir conducts research study using electrochemical cell with zine anode silver cathode und salt bridge with KCl as shown below. Before the experiment Damir measured the
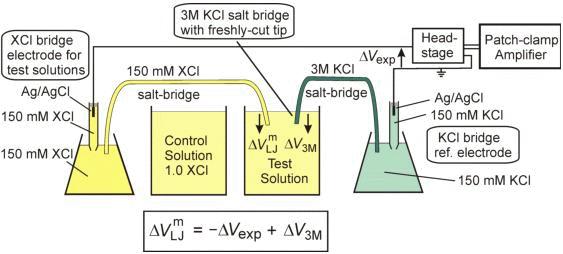
An optimised 3 M KCl salt-bridge technique used to measure and validate theoretical liquid junction potential values in patch-cl

A) Schematic of the micro-agar salt bridge. Three percent agarose in 3... | Download Scientific Diagram
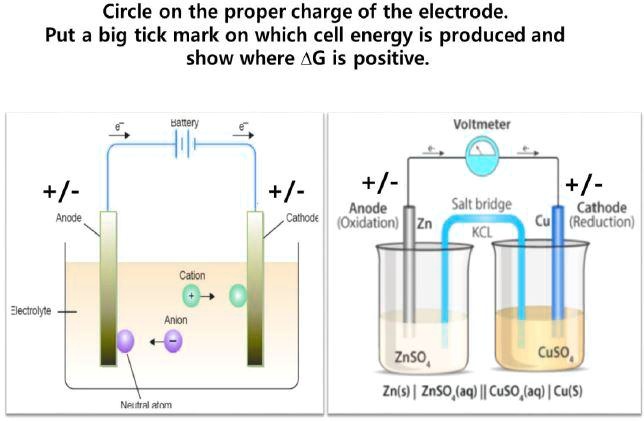
SOLVED: Circle on the proper charge of the electrode: Put a big tick mark on which cell energy is produced and show where AG is positive: Bacery Voltmeter +/- Anode +/- +/=

A Micro-agar Salt Bridge Electrode for Analyzing the Proton Turnover Rate of Recombinant Membrane Proteins. | Semantic Scholar

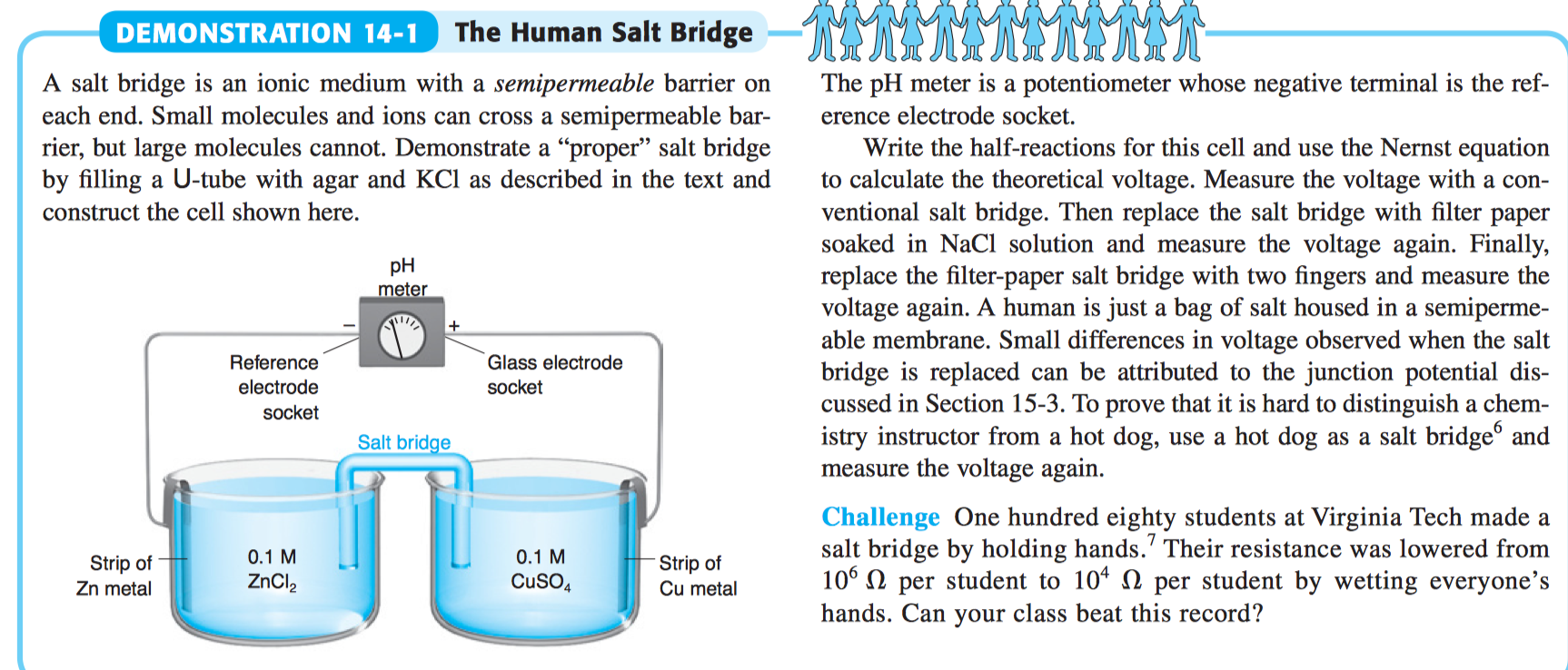
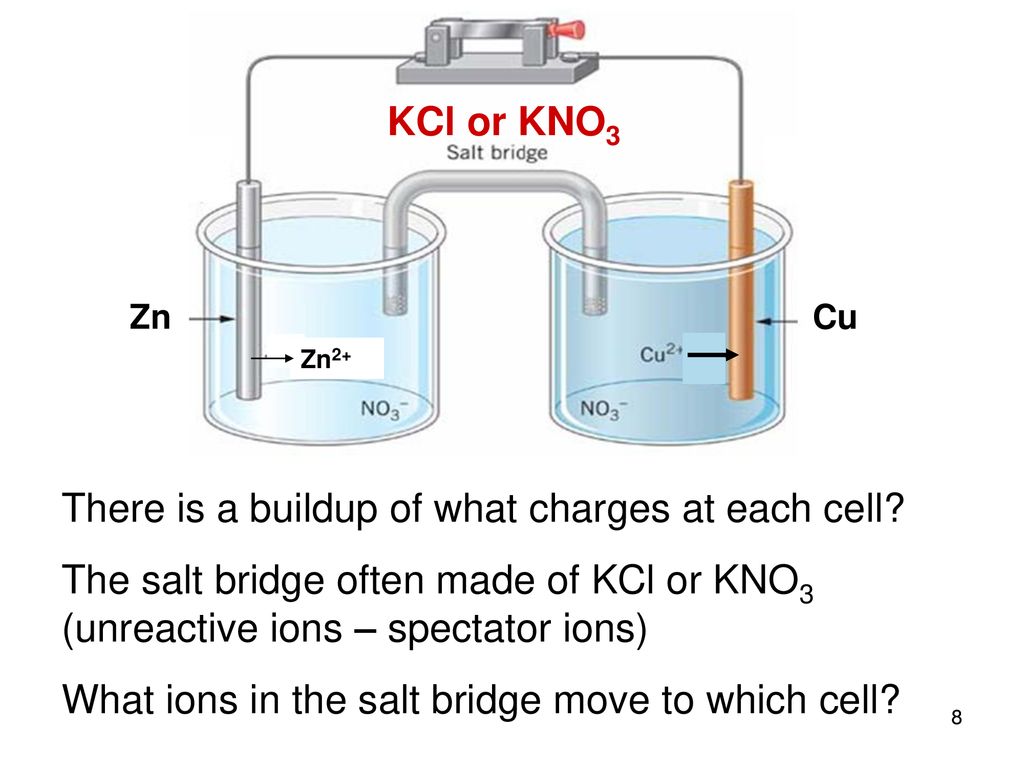
physical chemistry - Why is it important to use a salt bridge in a voltaic cell? Can a wire be used? - Chemistry Stack Exchange
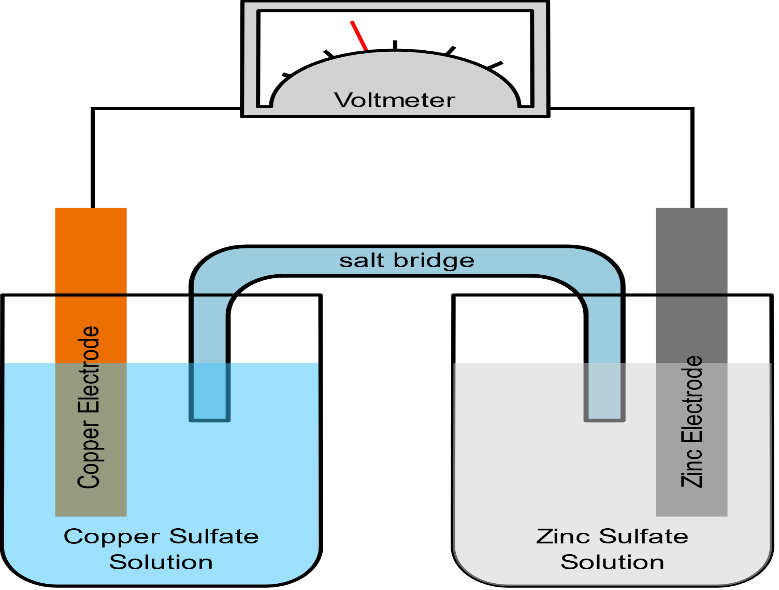
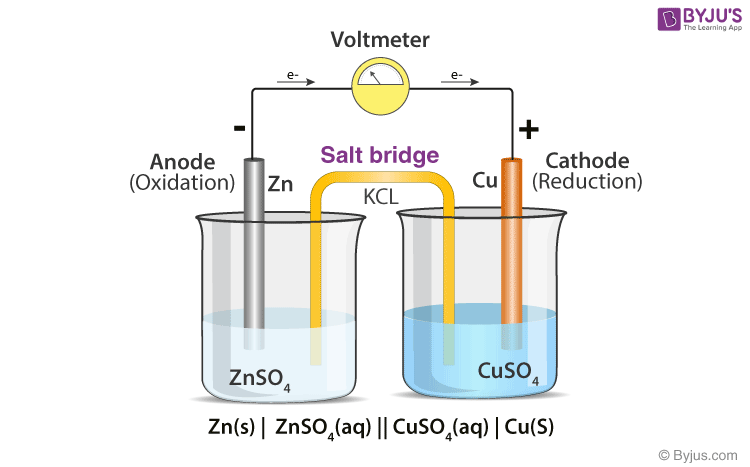
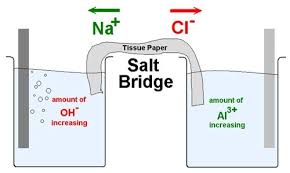
The function(s) of salt bridge in a cell is\/areA. It maintains standard electrode potential of cell constant which depends on several factors.B. It completes the electrical circuit.C. It departs both the solutions